Experimental drug could help prevent Alzheimer’s in certain population: study
An experimental drug has shown promise in preventing Alzheimer’s for people at higher risk of developing the disease.
That’s according to a new study from Washington University School of Medicine in St. Louis, where researchers ran a clinical trial of people with rare genetic mutations that almost “guarantee” future Alzheimer’s development, according to a press release.
The study included 73 people in their 30s, 40s and 50s who have the mutation, which causes an overproduction of amyloid in the brain.
PARKINSON’S CASES COULD DOUBLE GLOBALLY BY 2050, STUDY REVEALS
Amyloid, a protein that builds up in the brain and can interfere with cognitive function, is one of the hallmarks of Alzheimer’s.

An experimental drug has shown promise in preventing Alzheimer’s for people at higher risk of developing the disease. (iStock)
All participants had no (or very mild) cognitive decline, had a family history of Alzheimer’s, and were within 15 years before to 10 years after their expected age of developing symptoms, the release stated.
For 22 of the participants who received a drug called gantenerumab for eight years, their risk of developing symptoms was cut in half — from 100% to 50% — the researchers reported.
“What we do know is that it’s possible to at least delay the onset of the symptoms of Alzheimer’s disease and give people more years of healthy life.”
“There was no effect seen in those only treated for two to three years of treatment,” senior author Randall J. Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology at WashU Medicine, told Fox News Digital.
The findings were published in the journal The Lancet Neurology on March 19.

For 22 of the participants who received a drug called gantenerumab for eight years, their risk of developing symptoms was cut in half — from 100% to 50% — the researchers reported. (iStock)
Gantenerumab, a monoclonal antibody designed to target and remove amyloid plaques in the brain, was in development by Roche in Switzerland and its U.S. affiliate, Genentech.
Development was stopped in 2023, however, after Roche/Genentech’s own clinical trials found that the drug did not meet their “primary endpoint” for slowing cognitive decline in people with early symptomatic Alzheimer’s disease, according to the release.
HIGHER DEMENTIA RISK SEEN IN WOMEN WITH COMMON HEALTH ISSUE
“Everyone in this study was destined to develop Alzheimer’s disease and some of them haven’t yet,” said Bateman in the release.
“We don’t yet know how long they will remain symptom-free – maybe a few years or maybe decades. In order to give them the best opportunity to stay cognitively normal, we have continued treatment with another anti-amyloid antibody in hopes they will never develop symptoms at all,” he went on.

The hope is that if late-onset Alzheimer’s trials have similar results, prevention methods could ultimately be available to the general population, according to the researchers. (iStock)
“What we do know is that it’s possible to at least delay the onset of the symptoms of Alzheimer’s disease and give people more years of healthy life.”
The hope is that if late-onset Alzheimer’s trials have similar results, prevention methods could ultimately be available to the general population, according to Bateman.
DEMENTIA RISK COULD INCREASE WITH LOW LEVELS OF ESSENTIAL VITAMIN
“I am highly optimistic now, as this could be the first clinical evidence of what will become preventions for people at risk of Alzheimer’s disease,” he said. “One day soon, we may be delaying the onset of Alzheimer’s disease for millions.”
Howard Fillit, MD, co-founder and chief science officer at the Alzheimer’s Drug Discovery Foundation in New York, noted that the study shows for the first time that early treatment to clear the plaques before symptoms arise can delay the onset of Alzheimer’s — “similar to how we treat and prevent other chronic diseases.”

Although gantenerumab is no longer being developed, researchers are evaluating other anti-amyloid drugs — such as remternetug, which is made by Eli Lilly — to determine whether they may prevent Alzheimer’s disease. (iStock)
“We’ve entered into a new era of Alzheimer’s research where we can not only modify the course of the disease, but where prevention is possible with therapeutic intervention,” Fillit, who was not involved in the study, told Fox News Digital.
Potential limitations and risks
There were several main limitations to the research, Bateman told Fox News Digital.
The number of people was limited due to the rarity of Alzheimer’s disease caused by mutations, the use of external controls, and the fact that the study started with lower doses, he said.
TWO ALZHEIMER’S DRUGS HELP PATIENTS LIVE INDEPENDENTLY AT HOME FOR LONGER PERIODS
“Many of the participants are still cognitively normal and near or past their expected age of onset even after more than eight years of treatment, so the effects could be larger or smaller with continued treatment and follow-up,” Bateman noted.
The researchers noted that anti-amyloid medications like gantenerumab have been shown to cause amyloid-related imaging abnormalities (ARIA).
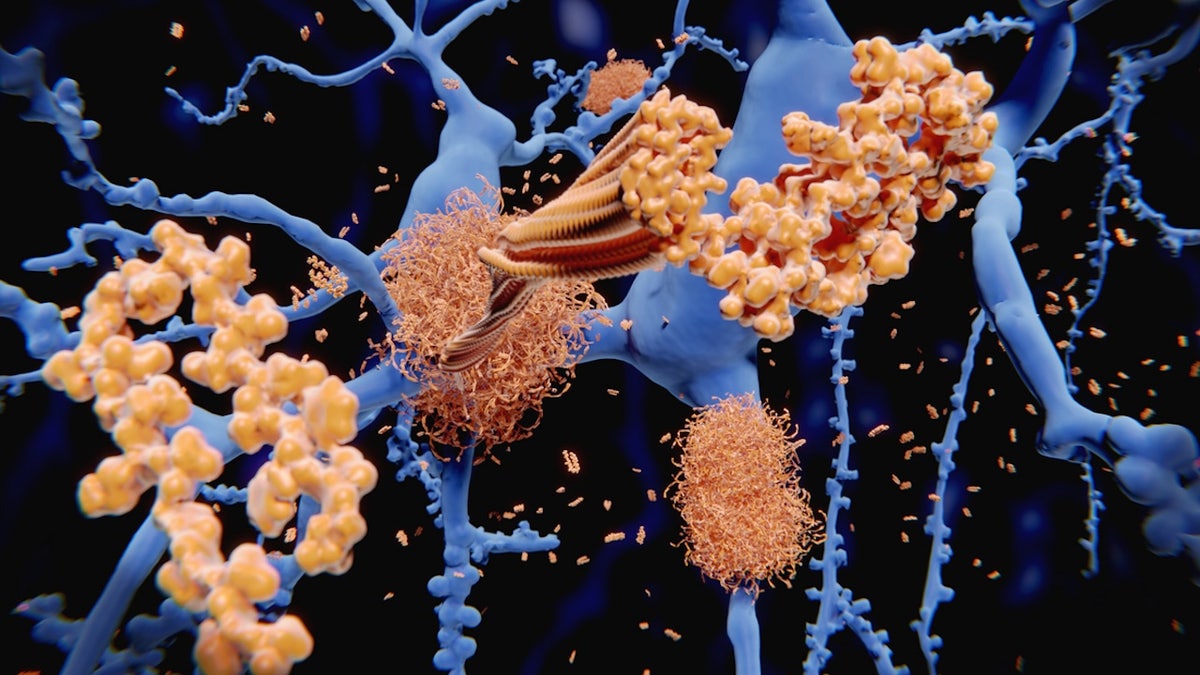
Amyloid, a protein that builds up in the brain and can interfere with cognitive function, is one of the hallmarks of Alzheimer’s. (iStock)
These appear on brain scans as “tiny spots of blood in the brain or localized swelling of the brain,” the release stated.
The majority of these side effects do not cause symptoms and resolve without treatment, but in rare cases ARIA can cause serious medical issues or can even be fatal.
This most recent study showed that 30% of participants experienced ARIA, likely due to the higher doses of the drug.
Although two participants had to stop using gantenerumab due to severe ARIA, there were no “life-threatening adverse events and no deaths,” the researchers noted.
“Overall, the safety profile of gantenerumab in the extension was similar to that in the original trial and in other clinical trials of gantenerumab,” they stated.
More research needed, experts agree
Dr. Chris Vercammen, a board-certified internal medicine physician who specializes in geriatrics and palliative care, said that while these initial findings are “encouraging,” more research is needed on the effects of these medications.
“Large, randomized trials, including diverse populations and individuals with late-onset Alzheimer’s, are needed to validate these early results and determine the full potential of these treatments,” Vercammen, who is also medical director at Remo Health in California, told Fox News Digital. (He was not involved in the new study.)
CLICK HERE TO GET THE FOX NEWS APP
“It’s important to note that this study’s design focused on high-risk individuals in the pre-clinical stage, and therefore does not provide sufficient data on the impact of these medications on later-stage Alzheimer’s.”
Fillit added that this new research opens the door for further exploration of treating preclinical Alzheimer’s.
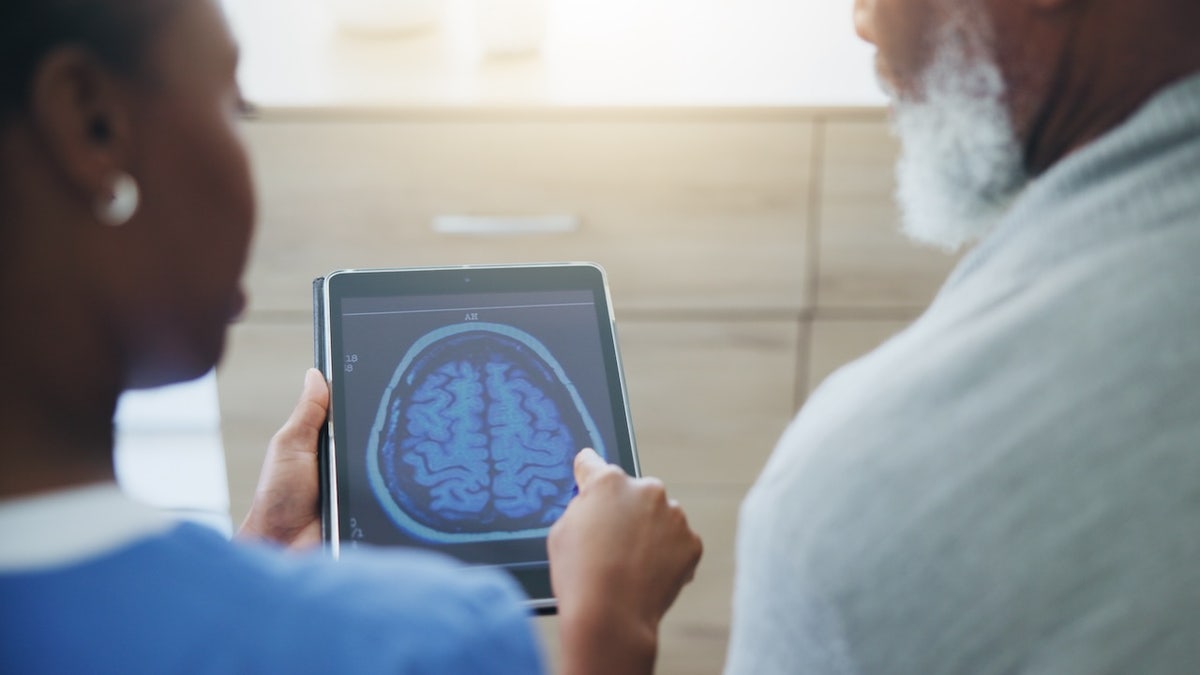
Anti-amyloid medications like gantenerumab have been shown to cause amyloid-related imaging abnormalities (ARIA), which appear on brain scans as “tiny spots of blood in the brain or localized swelling of the brain.” (iStock)
“We look forward to seeing the longitudinal data as well as further studies around this approach,” he told Fox News Digital.
“These efforts bring us one step closer to our ultimate goal of preventing the disease before it begins.”
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Although gantenerumab is no longer being developed, researchers are evaluating other anti-amyloid drugs — such as remternetug, which is made by Eli Lilly — to determine whether they may prevent Alzheimer’s disease.
“These efforts bring us one step closer to our ultimate goal of preventing the disease before it begins.”
“These rare families with mutations may wish to participate in ongoing trials,” Bateman told Fox News Digital.
“The older general population might be interested to know that there are ongoing trials in people with amyloid plaques to test this approach to determine if Alzheimer’s symptoms could be prevented.”
For more Health articles, visit www.foxnews.com/health
The study was funded primarily by the Alzheimer’s Association, GHR Foundation and the National Institutes of Health (NIH).
Fox News Digital reached out to Roche/Genentech for comment.